Fast, noninvasive, and inexpensive—capillary refill time (CRT) has become a crucial tool to assess perfusion at the bedside. CRT captures dynamic capillary response to hypoperfusion and holds prognostic value in various forms of shock. In this article, we review key clinical insights and explore CRT’s evolving role in practice.

What does CRT measure?
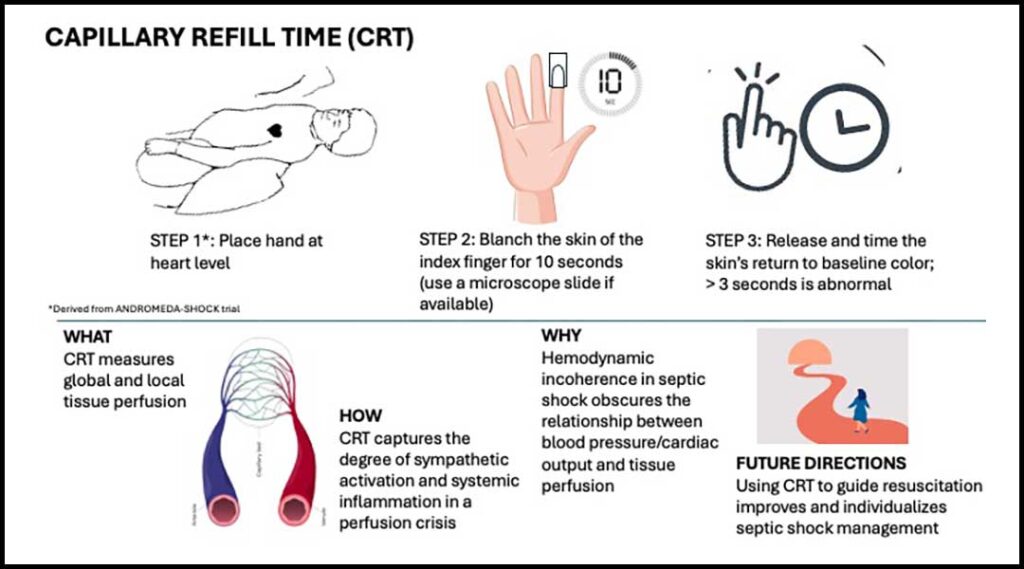
CRT measures two key components of perfusion: blood flow and capillary dysfunction. During a perfusion crisis, activation of the sympathetic system vasoconstricts peripheral vessels, leading to hypoperfusion of the skin and distal skeletal muscle. Unlike other organs, the skin is responsible for thermoregulation and has limited vasodilatory autoregulation to ischemia. CRT is a sensitive marker of sympathetic activation.
Additionally, in septic shock, microvasculature function decompensates due to capillary leak, glycocalyx disruption, and oxidative stress. The act of compression and reperfusion of the small vessels of the finger tests the integrity of the endothelium to local vasodilators, such as nitric oxide. While CRT and lactate are both markers of global hypoperfusion, CRT provides more responsive feedback on microcirculatory dysfunction in shock states.1
How to measure CRT
Several factors influence CRT measurements, including age, sex, ambient temperature, nail polish, and skin pigmentation. Additionally, user variability can impact accuracy, emphasizing the importance of standardized measurement techniques.

In the ANDROMEDA-SHOCK trial, CRT was measured by blanching the ventral surface of the index finger with a glass microscopic slide for 10 seconds before releasing.1 This method improves reproducibility and bypasses confounders such as nail polish.
Is CRT a marker of vital organ hypoperfusion?
CRT is an accurate measure of peripheral perfusion when compared with advanced microcirculation monitoring methods such as laser Doppler flowmetry and sublingual microscopy.2 Notably, impaired CRT has been linked to reduced perfusion in the intestines, kidneys, and liver among patients with septic shock.3
How does CRT relate to BP and cardiac output?
The association between CRT and principal factors in tissue perfusion like cardiac output (CO) and BP is not straightforward.4,5 In the presence of endothelial inflammation and microvascular dysfunction, resuscitation efforts to increase CO and BP, like fluid resuscitation and the use of vasopressors, may fail to improve CRT. This concept of hemodynamic incoherence underscores the need to prioritize peripheral perfusion markers and macrohemodynamic parameters when guiding septic shock resuscitation.6

CRT- vs lactate-guided resuscitation
Although in the ANDROMEDA-SHOCK trial CRT-targeted resuscitation led to similar 28-day mortality, it resulted in less fluid administration and earlier vasopressor de-escalation compared with lactate-targeted resuscitation.1 In addition, a Bayesian analysis of the data showed a strong likelihood of lower mortality and faster recovery of organ failure in the CRT-targeted group when compared with the lactate-targeted group.7
The upcoming ANDROMEDA-2 trial aims to advance CRT-guided resuscitation using a combination of multiple hemodynamic and perfusion parameters.8 The trial proposes an individualized algorithmic-driven approach to perfusion-guided resuscitation in septic shock. With growing evidence supporting its clinical utility, the role of CRT in perfusion-guided resuscitation continues to expand, paving the way for individualized management of critical illness.
References
1. Hernández G, Ospina-Tascón GA, Damiani LP, et al. Effect of a resuscitation strategy targeting peripheral perfusion status vs serum lactate levels on 28-Day mortality among patients with septic shock: the ANDROMEDA-SHOCK randomized clinical trial. JAMA. 2019;321(7):654-664. doi:10.1001/jama.2019.0071
2. Contreras R, Hernández G, Valenzuela ED, et al. Exploring the relationship between capillary refill time, skin blood flow and microcirculatory reactivity during early resuscitation of patients with septic shock: a pilot study. J Clin Monit Comput. 2023;37(3):839-845. Preprint. Posted online December 10, 2022. PMID: 36495360.
3. doi: 10.1007/s10877-022-00946-7Brunauer A, Koköfer A, Bataar O, et al. Changes in peripheral perfusion relate to visceral organ perfusion in early septic shock: a pilot study. J Crit Care. 2016;35:105-109. Preprint. Posted online May 12, 2016. PMID: 27481743.
4. doi: 10.1016/j.jcrc.2016.05.007Fage N, Moretto F, Rosalba D, et al. Effect on capillary refill time of volume expansion and increase of the norepinephrine dose in patients with septic shock. Crit Care. 2023;27(1):429. PMID: 37932812; PMCID: PMC10629142. doi: 10.1186/s13054-023-04714-0
5. Hernández G, Valenzuela ED, Kattan E,et al. Capillary refill time response to a fluid challenge or a vasopressor test: an observational, proof-of-concept study. Ann Intensive Care. 2024;14(1):49. PMID: 38558268; PMCID: PMC10984906.
6. doi: 10.1186/s13613-024-01275-5Bakker J, Ince C. Monitoring coherence between the macro and microcirculation in septic shock. Curr Opin Crit Care. 2020;26(3):267-272. doi:10.1097/MCC.0000000000000729
7. Zampieri FG, Damiani LP, Bakker J, et al. Effects of a resuscitation strategy targeting peripheral perfusion status versus serum lactate levels among patients with septic shock. A Bayesian reanalysis of the ANDROMEDA-SHOCK trial. Am J Respir Crit Care Med. 2020;201(4):423-429. doi:10.1164/rccm.201905-0968OC
8. Kattan E, Bakker J, Estenssoro E, et al. Hemodynamic phenotype-based, capillary refill time-targeted resuscitation in early septic shock: the ANDROMEDA-SHOCK-2 randomized clinical trial study protocol. Rev Bras Ter Intensiva. 2022;34(1):96-106. PMID: 35766659; PMCID: PMC9345585. doi: 10.5935/0103-507X.20220004-pt
